We operate in a full-service logic, to contribute to the success of your project.
From feasibility study, to design, through prototyping and production up to validation for certification.
Regulatory consulting
Assistance and support for CE marking, registration of medical devices on the database of the Ministry of Health and technical documentation management.
R&D & Design
Driving innovation through continuous research and development of materials and medical devices. A collaborative and optimized design and prototyping process.
Production
Merging scientific expertise and technology to create high-performance products that are fully compliant. Micro-extrusion and assembly in clean room.
Regulatory Training
New European Regulations (EU) 2017/745 and subsequent amendments, ISO 13485, ISO9001 and the Guidelines on the world of Medical Devices.
Company organization
People, technologies, technological know-how, process mapping, information management. To improve and reduce costs.
Quality systems
Consultancy for the implementation of Quality system for medical devices in compliance with the requirements set by target country regulations.
From the idea to the final product
We develop ideas and new solutions in the biomedical field, supporting the customer in all phases of the process.
From the analysis of the need to the certification of the products, from the validation of the devices regarding clinical aspects to the production and monitoring of the final product.
Find out how we work- Analysis of the needs
- Feasibility study
- Research and Development
- Prototyping
- Production
FAR.Medical Srl continuously improves the performance of its products, ensuring compliance with the most stringent standards that regulate the production of the medical sector. We are an ISO 13485 certified company for third party production and consultancy activities.
VIEW THE CERTIFICATE



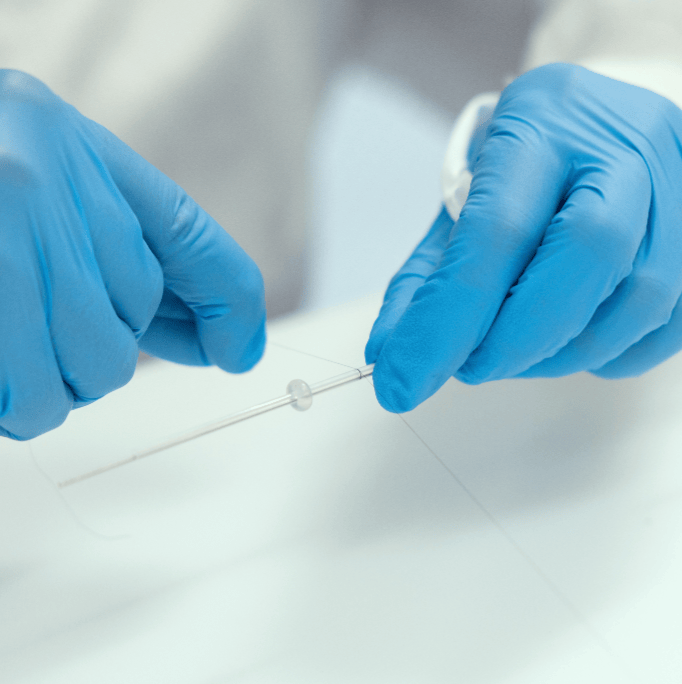
Our expertise in semi-finished product
Prototyping, extrusion and assembly in a clean room
FAR.Medical Solutions Srl has over a decade of experience in micro-extrusion for semi-finished products with external diameters starting from 0,40 mm.
- Design of dedicated tools for extrusion and micro-extrusuin
- Consulting for plastic materials and blends
- Consulting on screws and barrels with the aim to improve plasticization, stability and process standardization
Semi-finished devices
- Balloon tubes
- Blend tubes
- Braided Tube
- Bump-tubing (conical) Coming soon
- Multi Layer Coming soon
- Multi Lumen
- Single Lumen
- Tubes with radiovisible longitudinals markers (1 to 8 radiopaque lines) Coming soon
Regulatory consultancy for medical device manufacturers
We assist medical device manufacturers in preparing all the documentation necessary to undertake the certification process according to the regulatory standards MDR 2017/745 and IVDR 2017/746 .
The documentation is prepared for the design, validation and product testing phase, up to the drafting of the Technical File for medical devices and in vitro diagnostic medical devices of all Classes.
FAR.Medical Solutions Srl provides assistance and support for CE marking, registration of medical devices on the database of the Ministry of Health and technical documentation management
- Technical file for CE marking
- MDR Certification Process
- Product validation and testing
- Registration in the database of the Ministry of Health
- Registration in the European database (EUDAMED)
Success stories
Many biomedical and pharmaceutical companies believe in us, discover some of the projects we are very proud of.
Do you need to design and validate a biomedical product?
Contact us and let's talkEvents and news
To the newsMedica 2024 Fair
We will be present as exhibitors at the MEDICA 2024 Fair, one of the most important international fairs dedicated to […]
Read more